What is ECTD publishing and why it's a key part of regulatory affairs
- Tom Marsden
- Mar 7
- 2 min read
Updated: Mar 10
In the world of pharmaceuticals, getting a drug to market isn’t just about research and innovation—it’s about regulatory compliance. That’s where eCTD publishing comes in. The Electronic Common Technical Document (eCTD) is the globally accepted format for submitting regulatory dossiers to health authorities like the FDA, EMA, MHRA, Health Canada, and more. It’s the digital backbone that allows pharmaceutical companies to get approvals for new drugs, biologics, and medical devices, ensuring they can be sold across the world.

Why is eCTD Publishing So Important?
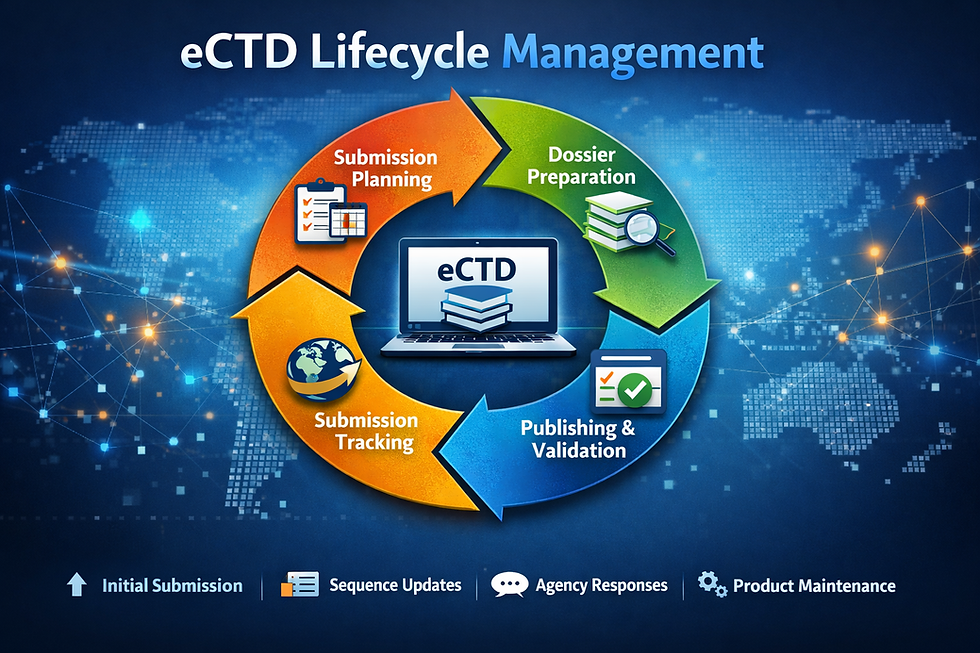
Regulatory agencies demand precision, consistency, and compliance. Without a properly formatted eCTD submission, approvals can be delayed—or worse, rejected. eCTD publishing ensures that:
Regulatory authorities can easily review and track your submission across its lifecycle
All documents are properly linked, structured, and validated to meet technical requirements
Companies can manage changes efficiently with seamless updates and amendments
Whether it’s a new drug application, a variation, or a renewal, eCTD publishing is the key to keeping products on the market and in the hands of patients who need them.
Breaking Down the eCTD: The 5 Modules That Shape Every Submission
The eCTD is structured into five core modules, each playing a critical role in regulatory approval. Here’s how they work:
Module 1: Regional Administrative Information
This module contains region-specific documents, such as cover letters, application forms (EAFs), and regulatory correspondence.
Each health authority has its own specific requirements, making expertise in this area essential for compliance.
Module 2: Overview & Summaries
Provides high-level summaries of the quality, nonclinical, and clinical data contained in the full submission.
Designed to give reviewers a concise and well-structured overview before diving into the detailed data.
Module 3: Quality (Pharmaceutical & Manufacturing Data)
Contains comprehensive details on drug substance and drug product manufacturing, stability, and control.
Critical for proving that a pharmaceutical product is consistently produced with high quality.
Module 4: Nonclinical Study Reports
Includes data from animal studies, covering toxicology, pharmacology, and safety evaluations.
Demonstrates that the product has been thoroughly tested for potential risks before human trials.
Module 5: Clinical Study Reports
The heart of every regulatory submission—clinical trial data proving the drug is safe and effective for patients.
Contains results from Phase 1–4 clinical studies, including efficacy, safety, and risk-benefit analyses.
Why Trust eCTD Pharma for Expert eCTD Publishing?
At eCTD Pharma, we don’t just publish eCTDs—we perfect them. Our expert team ensures that every submission is:
Compliant with global regulatory requirements
Flawlessly validated to prevent rejection
Optimized for fast, efficient approval
With decades of experience handling complex, high-stakes submissions, we help pharmaceutical and biotech companies bring their products to market faster and more efficiently.
Let’s Get Your Submission Right the First Time
Regulatory success starts with flawless eCTD publishing. Partner with eCTD Pharma today to ensure your submission meets every requirement, every time.
Contact us now to discuss your next eCTD submission!



Comments