Mastering eCTD Lifecycle Management: Why Outsourcing Regulatory Submissions with ECTD Pharma Is Your Strategic Advantage
- Dec 20, 2025
- 6 min read
Regulatory authorities such as the US FDA, EMA, MHRA, and others require meticulously structured dossiers that adhere to strict electronic standards — notably the Electronic Common Technical Document (eCTD). Getting it right isn’t optional: it determines whether your application is accepted, delayed, or outright rejected.
Yet regulatory submissions aren’t a one-and-done event. They encompass a full submission lifecycle, from initial filing and approval through variations, renewals, health authority queries, post-approval changes, and long-term compliance. Managing this lifecycle effectively is a specialized discipline — one that requires deep regulatory expertise, efficient processes, and cutting-edge technology.
That’s where ECTD Pharma excels. As a trusted partner for global regulatory submissions and eCTD lifecycle management, we help life sciences organizations reduce risk, save time, and focus on innovation — not paperwork. In this comprehensive blog, we’ll explore what lifecycle management really involves, why outsourcing regulatory submissions makes strategic sense, and how ECTD Pharma delivers measurable advantages.
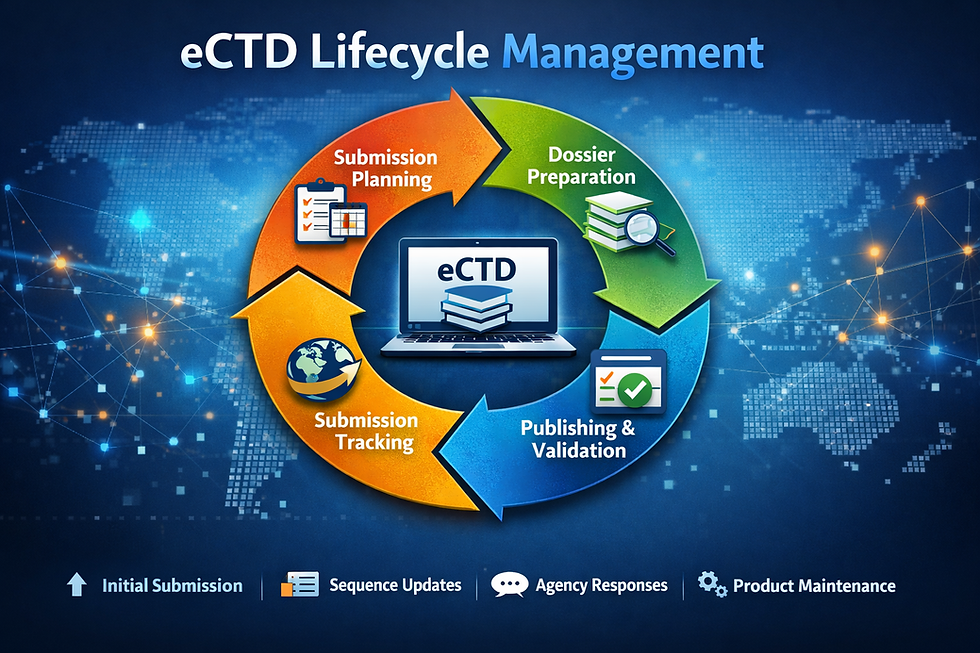
What Is eCTD Lifecycle Management?
At its core, eCTD lifecycle management refers to the continuous process of maintaining, updating, and submitting regulatory dossiers throughout the life of a product. It begins with the initial submission and continues with every post-approval update — including amendments, safety reports, variations, renewals, labeling changes, and responses to health authority requests.
Unlike a single submission event, lifecycle management treats regulatory activity as an ongoing strategic function. Each update becomes a new “sequence” or iteration of the dossier that must integrate seamlessly with prior versions, adhere to technical standards, and remain compliant with regional expectations.
Managing this lifecycle becomes especially complex when operating across global markets. Differences in formatting standards, submission portals, metadata requirements, and regional expectations make regulatory competence and consistency paramount.
The Importance of Strong Lifecycle Management
Poor lifecycle management jeopardizes your regulatory roadmap in multiple ways:
❌ Increased Errors and Rejections
Minor glitches in formatting, metadata, hyperlinking, or sequence continuity can trigger technical rejections from regulators — leading to costly delays.
❌ Fragmented Document History
Without robust storage and version tracking, it becomes difficult to trace the document history needed for future submissions and regulatory inspections.
❌ Rampant Inefficiencies
Manual or ad-hoc approaches consume internal resources and slow down approval timelines — particularly when teams lack specialized eCTD expertise.
❌ Compliance Risk
Health authorities frequently update their submission expectations. A misstep or outdated submission can trigger compliance gaps that affect approvals and post-market obligations.
Lifecycle management isn’t just an operational task — it’s a strategic imperative that ensures your regulatory history is coherent, compliant, and submission-ready at all times.
Why Outsourcing Regulatory Submissions Makes Sense
Many companies — especially smaller biotechs, mid-sized pharma, and emerging life sciences teams — struggle with the internal resourcing required for effective lifecycle management. Building an in-house regulatory submissions team is expensive, time-consuming, and hard to scale. That’s where outsourcing becomes a strategic decision with high ROI. Ectd Pharma
Here are core reasons why outsourcing regulatory submissions and lifecycle management is smart business:
1. Specialized Expertise and Regulatory Intelligence
Regulatory guidelines change regularly — and they vary by agency. Outsourcing gives you access to specialists who understand evolving standards, global variances, and best practices for structuring regulatory submissions.
Expert regulatory consultants:
Know the differences between FDA, EMA, and MHRA expectations
Apply optimal eCTD granularity
Anticipate regulatory timelines and checkpoints
This level of insight cuts risk and accelerates timelines.
2. Meticulous Technical Accuracy
Regulators expect technical perfection. Outsourcing brings advanced quality controls such as:
PDF bookmarking and hyperlinking
XML backbone structuring
Metadata validation
Checksum integrity
These elements — often overlooked in internal workflows — are critical to acceptance. Ectd Pharma
3. Time and Cost Efficiency
Hiring, training, and retaining an internal publishing and regulatory team is expensive and inefficient, especially for intermittent submission workloads. Outsourcing offers:
Flexible scaling based on workload
Cost savings without hiring full-time staff
Predictable project pricing
This frees your internal teams to focus on core activities like research, clinical operations, or commercial strategy.
4. Advanced Technology and Submission Platforms
Leading outsourcing partners leverage state-of-the-art technology — often without you having to license or maintain it yourself. ECTD Pharma, for example, uses secure in-house publishing platforms and tools tailored for global regulatory compliance.
Your submissions benefit from:
Validated publishing software
Secure cloud-based storage
Real-time collaboration systems
Direct portal upload capabilities
This level of infrastructure is costly to replicate internally.
5. Reduced Risk, Faster Approvals
Ultimately, outsourcing increases your chances of first-time acceptance. Precision in submissions — combined with expert lifecycle support — reduces regulatory queries, rejections, and costly delays. Faster approval timelines accelerate your time to market. Ectd Pharma
Why Choose ECTD Pharma: Key Benefits
With so many regulatory consultancies in the market, why partner specifically with ECTD Pharma?
Here’s how we stand out:
✔️ Global Experience and Proven Track Record
ECTD Pharma has a long history of supporting regulatory submissions for companies across the world, from small biotechs to established pharma organizations. With thousands of successful submissions and a 100% acceptance rate, our experience speaks for itself.
✔️ End-to-End Lifecycle Management
Our services cover:
Initial submission preparation
eCTD publishing and compilation
Validation and quality control
Direct submissions via FDA ESG, EMA, MHRA portals
Post-approval updates (variations, renewals, annual reports)
Responses to health authority queries
Secure submission storage and version control
This continuous lifecycle support ensures regulatory histories are complete, compliant, and up to date. Ectd Pharma+1
✔️ Personalized Regulatory Partnerships
At ECTD Pharma, you work with experienced consultants — not faceless teams. From onboarding to submission and beyond, you receive hands-on guidance and transparency throughout the process. Ectd Pharma
✔️ Direct Submission Capabilities
We can submit your application directly through regulated portals like:
FDA Electronic Submissions Gateway (ESG)
EMA eSubmission Gateway
MHRA Submissions Portal
This direct access streamlines processing and reduces time to receipt. Ectd Pharma
✔️ Advanced Technology and Secure Systems
ECTD Pharma’s infrastructure — including in-house publishing software and secure cloud collaboration (e.g., SharePoint) — ensures your data is safe, accessible, and organized for future lifecycle updates. Ectd Pharma
✔️ Cost-Effective and Scalable Solutions
Whether you need a one-off submission, ongoing lifecycle support, or full outsourcing, our solutions are designed to match your project scale — without unnecessary spend.
What Clients Say About ECTD Pharma
“ECTD Pharma has been an invaluable partner for our regulatory submissions. Their team provided expert eCTD document preparation and ensured our FDA and EMA submissions were fully compliant…” — Simon, Project Manager
This testimonial reflects our commitment to quality, responsiveness, and technical excellence in regulatory submissions and lifecycle support.
How Our Process Works: A Strategic, Six-Step Approach
ECTD Pharma follows a structured, transparent workflow designed to minimize risk and maximize regulatory success:
Strategic Onboarding & Planning – Define regulatory scope, timelines, and requirements.
Document Preparation – Review source documents for completeness and compliance.
Compilation – Structure and organize modules into eCTD format.
Publishing & Validation – Use advanced tools to publish and check for technical perfection.
Submission or Delivery – Directly submit or deliver a submission-ready package.
Lifecycle Management & Ongoing Support – Maintain submission history and handle future updates.
This process ensures your regulatory submission is not only compliant but optimized for acceptance.
SEO-Focused Summary: Why Outsourcing Regulatory Submissions Matters
The life sciences industry increasingly recognises that outsourcing regulatory submissions is no longer a luxury but a strategic necessity. It reduces operational burden, mitigates regulatory risk, and ensures submissions adhere to stringent global standards. Consultants bring subject-matter expertise, operational efficiency, advanced technology, and cost savings that internal teams may struggle to match. News-Medical
By entrusting your regulatory submissions and lifecycle management to ECTD Pharma, you gain a reliable partner dedicated to excellence, precision, and regulatory success — every step of the way.
Final Word: Partner With ECTD Pharma for Regulatory Submission Success
Regulatory submissions define your path to market. They impact timelines, costs, and ultimately, patient access. Effective eCTD lifecycle management ensures your dossier remains compliant, coherent, and ready for every regulatory requirement.
Outsourcing regulatory submissions to ECTD Pharma helps you:
Reduce errors and risk
Save time and internal resources
Navigate global regulatory complexities with confidence
Focus on innovation — not paperwork
If you’re ready to elevate your regulatory submission process, accelerate approval timelines, and simplify lifecycle management, ECTD Pharma is here to help.
👉 Book a consultation today and let us handle your regulatory journey with the expertise, care, and precision your products deserve.




Comments